Abstract
Introduction The survival rate for children with B-cell acute lymphoblastic leukemia (B-ALL) now approaches 90%. However, given the poor prognosis of those who relapse, B-ALL remains a leading cause of cancer related mortality in children. To discover underlying mechanisms of treatment failure, previous genomic profiling has identified somatic alterations in 12 genes that drive treatment resistance. However, these studies were underpowered to detect association between these alterations and known genetic subtypes (e.g., hyperdiploid, ETV6::RUNX1, TCF3::PBX1, etc.) that would indicate cooperativity in driving clonal evolution.
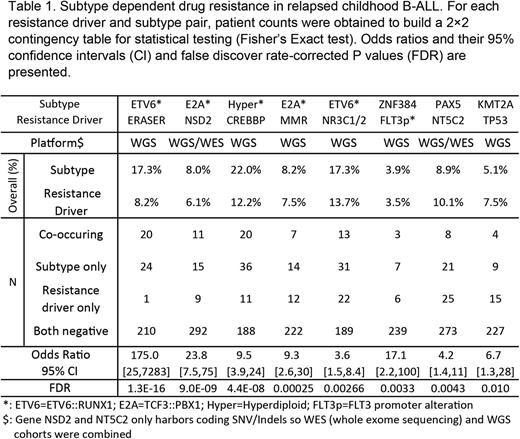
Results We performed whole genome sequencing (WGS) of diagnosis/remission/relapse specimens of 181 B-ALL cases (age 0-18 years) enrolled on a recently completed clinical trial for relapsed B-ALL (AALL1331). Somatic aberrations including SNV, Indel, structural variations (SV), copy number variations (CNV) were identified from WGS and fusion genes were detected from RNAseq data. By integrating these cases with 146 previously published cases (totaling N=327 with WGS/WES; N=255 with WGS), we aimed to identify novel resistance drivers and to discover associations between genetic subtypes and resistance drivers. Here, we report two novel somatic alterations to be enriched in relapsed B-ALL (Table 1), an intergenic deletion in chr1 (named ERASER) and FLT3 promoter alteration (which results in overexpression of FLT3). Importantly, we also discovered statistically significant (with FDR corrected P value<0.2) associations between resistance drivers and subtypes that confirmed previously known associations such as NR3C1/NR3C2 in ETV6::RUNX1 and FLT3 promoter alteration in hyperdiploid ALL. We also discovered novel associations including NSD2 in TCF3::PBX1, CREBBP in hyperdiploid, ERASER in ETV6::RUNX1, TP53 in KMT2Ar, mismatch repair deficiency (MMR) in TCF3::PBX1, NT5C2 in PAX5 alt and DUX4, and FLT3 promoter alteration in ZNF384 subtypes. In addition to these novel findings, our data also revealed high frequency nested P2RY8::CRLF2 fusions in other subtypes, which was validated using single cell sequencing.
Conclusion Together, our study features NGS of the largest cohort of relapsed childhood ALL samples, identifies novel resistance markers for ETV6::RUNX subtype, and uncovers novel associations between resistance drivers and subtypes. These finding may shape future clinical management of childhood ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal